Table of Contents
What To Know About Today's Ethanol Fuels
The blending of ethanol into gasoline across the nation is now a common practice due to recent EPA mandates for 10% and 15% ethanol blends. Ethanol causes major issues for consumers, who face loss of mileage, storage issues and a tendency for ethanol to corrode plastic and fiberglass tanks and parts, especially in marine applications. Homeowners who have to use ethanol fuels in their lawn and garden equipment find serious damage being done. Here’s what you need to know about what ethanol does, why it does it, and what you can do about it.
Why Ethanol?
In many states, it’s hard to find a gas station that isn’t selling at least 10% ethanol in their gasoline; you see the warning stickers on all of the pumps. Most people don’t really know why it’s put into gasoline; they just know they may have heard bad things about it. Ethanol is classified as an “oxygenate”, meaning it increases the oxygen content of the fuel that it is blended into. The EPA has historically used government mandates (as allowed by the Clean Air Act) to force the introduction of oxygenates into gasoline, as a way to help reduce emissions like carbon monoxide and improve urban air quality.
Following several previous pieces of legislation, the EPA really changed the game in 1992, when MTBE (which had already been used in the 1970s as an anti-knock agent in gasoline) began being blended into gasoline to help cut harmful emissions. At its peak in 1999, 200,000 barrels (8.4 million gallons) per day of MTBE were being produced, all being added to gasoline at a 10% treat ratio. Unfortunately, scientists began to find evidence that MTBE was linked to ill-health effects, and also found it easily contaminated ground water; these led to its widespread withdrawal from the market. This is what allowed ethanol to displace MTBE as the dominant oxygenate of choice to blend into gasoline which satisfied these EPA emissions requirements.
As of 2012, we have 15% ethanol infiltrating the nation’s fuel supply. 15% ethanol, or E15, was approved by the EPA a couple years earlier, but only for 2007 model vehicles and later.
What’s Good About Ethanol?
As with many things in life, not everything about ethanol is bad.
Lower Emissions
To be sure, ethanol imparts some advantageous qualities when blended into gasoline. First and foremost are reduced emissions. These may not be so important to the average consumer (unless they are concerned about going green),but this is the advantage the EPA and environmental scientists like. Ethanol blended into gasoline at a 10-85% ratio makes fuel that produces lower levels of harmful urban air pollutants than pure gasoline, which brings positive effects on smog and pollution levels in urban areas that may have traditionally struggled with this problem. These urban areas have a financial incentive to care about the problem, because areas out of compliance with Federal air quality standards (hence, the EPA’s jurisdiction applies here) can be at risk of losing access to important federal funds for the many things they use federal money to pay for.
Higher Octane
Oxygenates like ethanol and MTBE already were in historical use before the 1992 Clean Air Act as octane improvers. Pure ethanol has an octane rating of 113, while E10 blends have the octane rating listed at the pump, which is usually the same as regular or premium gasoline. Unfortunately for the consumer, it is likely because, despite the ethanol additive having a high octane rating, the fuel blender uses a lower octane base gasoline in order to end up with the same octane rating in the E10 blend as they had before. So the consumer doesn’t really get an added octane benefit in an E10, despite the ethanol fraction having a higher octane rating.
What’s Bad About Ethanol?
Ethanol Solvency and the Damage It Causes
Boat owners in the northeast can readily testify how ethanol blends up to E85 attach and dissolve rubber and plastic parts, even fiberglass fuel tanks. Ethanol has always been an excellent solvent and unfortunately, this is not a good thing for engines and fuel delivery systems which rely on rubber and plastic parts for their function. The ethanol fuel softens and dissolves rubber and plastic parts wherever it touches. This is not as big a problem in modern cars and trucks, but it is a problem in boats and especially in small equipment (including lawn and garden equipment). Many homeowners put their mowers away and come back later to find them inoperable because the ethanol fuel has destroyed the fuel line.
What’s more, these dissolved rubber and plastic resins have to go somewhere. These settle out in other parts of the fuel system and engine, including valves and injectors. And when you have deposits in places they’re not supposed to be, the engine doesn’t run right and you get poor performance all around.
Loss of Mileage – You Use More Fuel and Pay More
Loss of mileage from the use of ethanol blends results from the ethanol molecule containing less energy value than gasoline. The energy value in petroleum fuels is a function of the number of carbon bonds in the molecule. Gasoline molecules are much longer with more carbon bonds than the small ethanol molecules, so you have less energy potential in that blended fuel. Pure ethanol has a gross BTU value of 35% less than the equivalent amount of gasoline.
However, most cars don’t run on pure ethanol running on higher than 15-20% ethanol concentration can cause engine damage because the engine has to be adjusted to account for the differing combustion property of that concentration.. The commonly found E10 blend only has 10% ethanol, so the actual drop in energy value is more along the lines of 3.5%-5.0%.
Ethanol Dissolves Water – This Is Not A Good Thing
Pure ethanol has a strong ability to dissolve more water from the atmosphere around it than regular gasoline does. Some characterize it as ethanol attracting or pulling water in from the air but this isn't strictly correct. What they are referring to, no matter how they describe it, is that ethanol enhances the fuel's ability (and tendency) to dissolve more water.
This affinity is so strong that chemical producers cannot even sell 100% pure ethanol – it is always 99.8% or less because there will always be at least a tiny bit of water. As you may expect, the attraction of water is an even bigger problem for marine users of E10-E85 than it is for on-road drivers.
When water accumulates in a fuel or storage tank, it sinks to the bottom of the tank because water is heavier than fuel. It then contributes to a whole host of fuel problems and issues, including:
2-Cycle Engine Damage from Lack of Lubrication – Can Be Deadly
Owners of 2-cycle engines know they rely on lubrication from the fuel-oil mixture. When ethanol absorbs water, this blocks the lubrication from fully bonding with the metal surfaces, leading to engine damage.
Phase Separation
Phase Separation means the ethanol ‘phase’ separates from the gasoline ‘phase’ and results in two layers of two different compounds, instead of a homogenous mixture of gasoline and ethanol. At this point the ethanol will sink below the gasoline phase and mix with any more accumulated water, giving an ethanol-water phase mixture. This leads to other problems, including……
Loss of Octane In The Fuel
When the ethanol separates from gasoline, it causes a loss of 2-4 octane points in the fuel mixture; in effect, as it separates, it drags the octane value of the gasoline. Not just because the ethanol itself supplies octane value (which is now settling at the bottom of the tank), but also because the ethanol can solvate and dissolve certain kinds of molecules in the gasoline that themselves contribute to the fuel’s octane rating. When the ethanol drops out of the solution, it carries some of these molecules away. So an 87-octane fuel that separates can have its octane rating drop to 83-84, which is unsatisfactory for most vehicles and will cause performance issues.
Potential for Equipment Damage from Phase Separation
An ethanol blend that has separated will have the ethanol and water mixture settled at the bottom of the tank, where the fuel line is. The fuel line potentially can suck this water mixture up into the combustion chamber, where it will burn like an overly lean mixture (lean = not enough gasoline). Because it is not mostly gasoline now, burning this kind of fuel gives real potential for valve damage.
Degrading of Fuel Quality and Subsequent Deposit Buildup
Water is one of the impurities that will accelerate oxidation reactions in any petroleum-based fuel, whether gasoline, diesel, biodiesel, or ethanol blends. Oxidation reactions are responsible for fuel stratification and the fallout of heavy ends from the fuel mixture. These heavy ends can build up in the bottom of a fuel storage tank, and when they are injected as fuel, they do not burn like pure fuel but will leave deposits in all parts of the combustion system – the combustion chamber, valves, and fuel injectors. At best, you get raised emissions to the catalytic converter, rough running, and poor engine performance, while at worst you get a drop in mileage.
Ethanol also reacts with leftover MTBE, which used to be used as an octane improver. Some states have banned MTBE but you can still find it in marina fuel and off-road gasoline in other states like Florida. If the fuel handler mixes E10 with gasoline having MTBE, a brown gel sludge can form. This issue has been widely reported among boats and other users, who may not know what it is.
Conclusion
In exchange for becoming more “green”, consumers face a trade-off with certain problems that ethanol blends can cause in their vehicles and boats. Subsequently, there is a substantial market for additives out there to treat ethanol blends and blunt these problems. Some of them are better than others. The best ethanol additives will contain combustion improvers to blunt the mileage drop, detergents to clean out deposits and any dissolved resin buildup, an ingredient to disperse and control water buildup, and an ingredient to protect rubber and plastic parts from ethanol solvency. Beware of products that make outrageous claims and guarantees – if it seems too good to be true (guaranteed 35% mileage increase? Really?), it very likely is too good to be true.
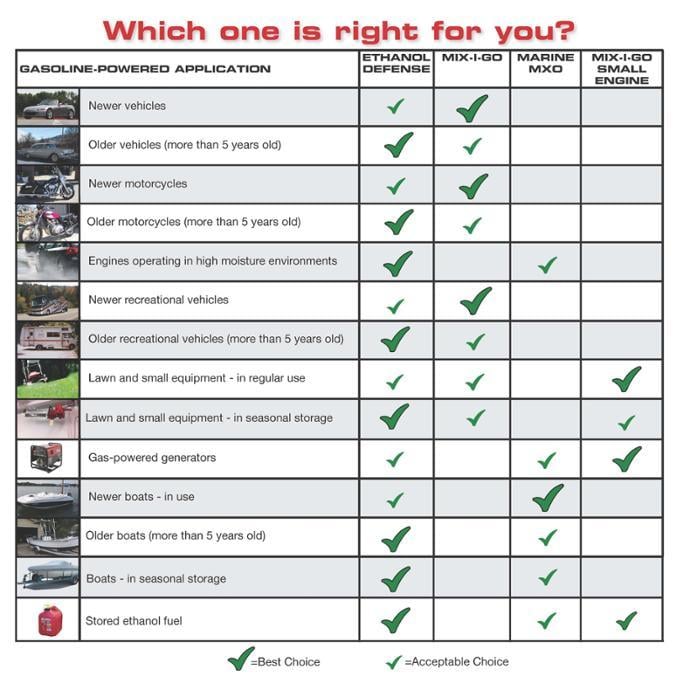
It’s not surprising so many people are holding up fuel treatments all claiming to be the best. If you’re stumped about what to use among all the treatments out there, a good idea may be to go with a treatment with a longer track record of success. For example, Bell Performance has been in business since 1909. Its fuel treatments Ethanol Defense, Mix-I-Go, Marine MXO, and Mix-I-Go Small Engine have such a track record and are all designed to address these ethanol problems in cars, trucks, boat, and small engines.